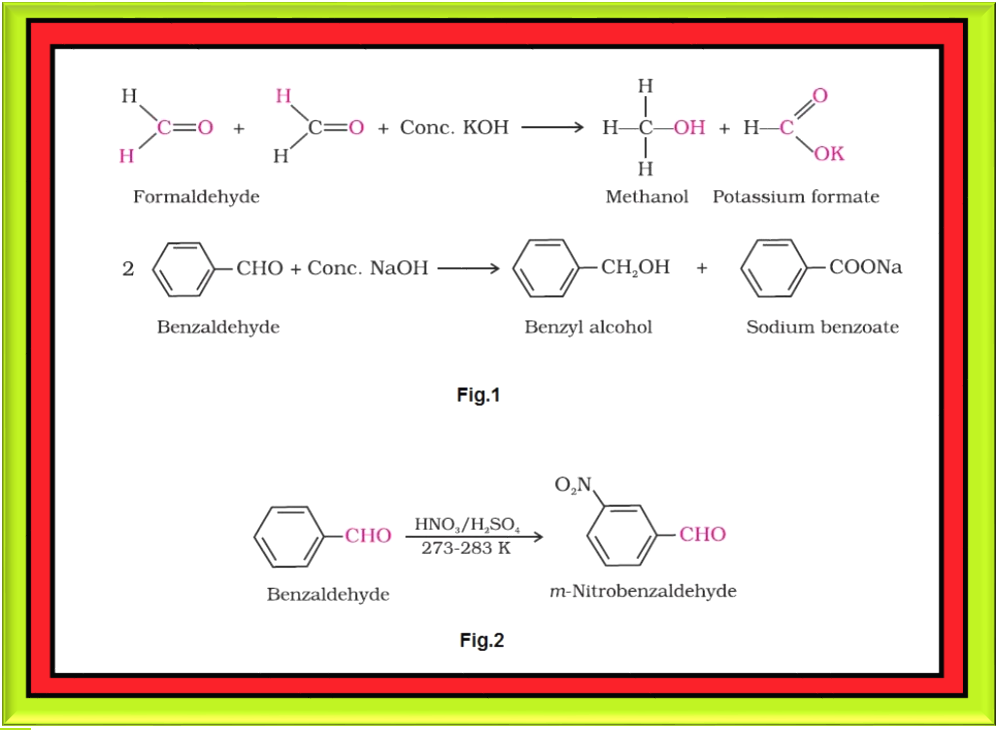
Cross Aldol Condensation :
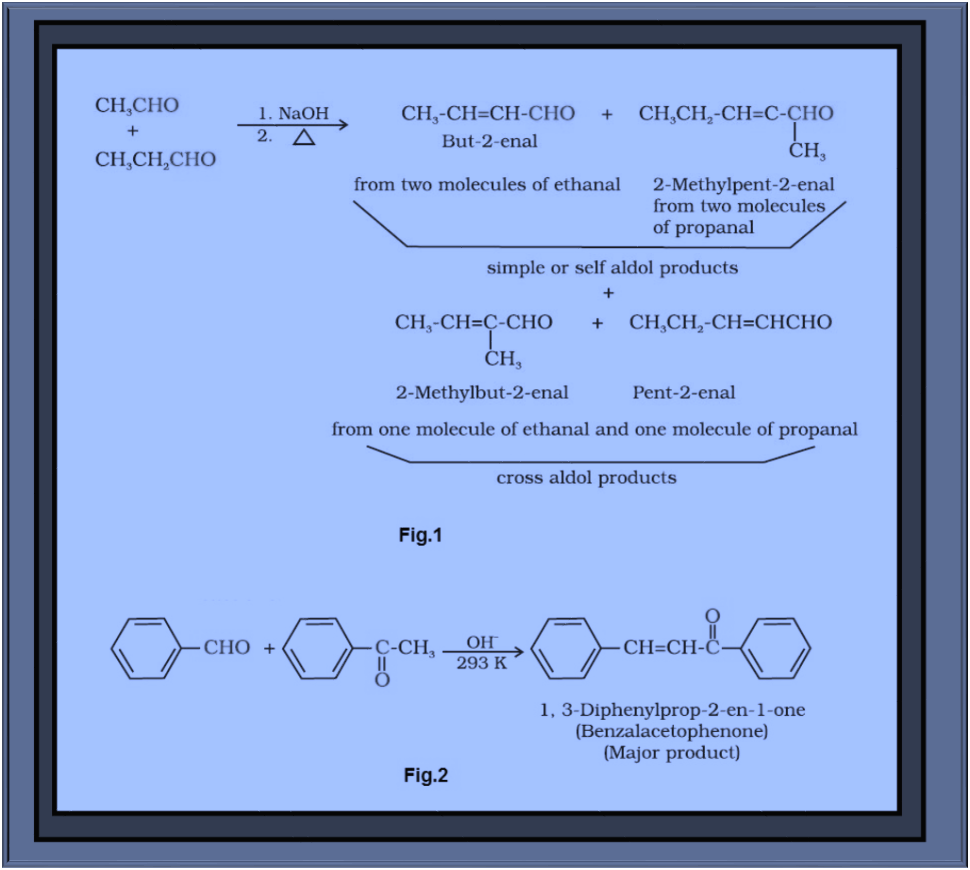
`=>` When aldol condensation is carried out between two different aldehydes and/or ketones, it is called cross aldol condensation.
`=>` If both of them contain `color{red}(α)`-hydrogen atoms, it gives a mixture of four products.
`=>` This is illustrated in fig.1 by aldol reaction of a mixture of ethanal and propanal.
`=>` Ketones can also be used as one component in the cross aldol reactions. See fig.2.
`=>` If both of them contain `color{red}(α)`-hydrogen atoms, it gives a mixture of four products.
`=>` This is illustrated in fig.1 by aldol reaction of a mixture of ethanal and propanal.
`=>` Ketones can also be used as one component in the cross aldol reactions. See fig.2.